Catalytic Addition of Nitroalkanes to Unactivated Alkenes via Directed Carbopalladation
Abstract
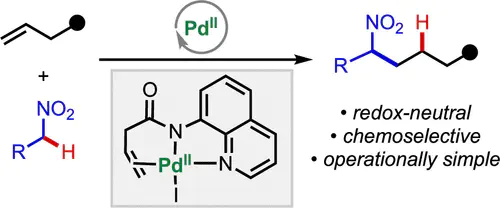
We report a redox-neutral catalytic coupling of nitroalkanes and unactivated alkenes that proceeds by a directed carbopalladation mechanism. The reaction is uniquely enabled by the combination of PdI2 as the precatalyst and HFIP solvent. Structurally complex nitroalkane products, including nitro-containing carbo- and heterocycles, are prepared under operationally convenient conditions without the need for toxic or corrosive reagents. Deuterium labeling experiments and isolation of a catalytically relevant intermediate shed light on the reaction mechanism. By taking advantage of different catalytic activation modes, we demonstrate orthogonal methods for site-selective functionalization of a polyfunctional nitroalkyl ketone. Density functional theory (DFT) calculations show that the carbopalladation transition state is stabilized by a Na···I interaction and H···I hydrogen bond with HFIP.